Keywords: CENTER-TBI, TBI registry, TBI
Authors: Irina Vlad, Silvina Ilut
As we have indicated in our first article “CENTER-TBI: the making-of the main TBI registry in Europe – Part 1” a follow-up on important lessons from the CENTER-TBI project will be addressed further on, as well as the role of this endeavor for global TBI research and clinical care. Therefore, here we are tackling the second part of this very interesting project.
Lessons from CENTER-TBI
A significant result regarding TBI that CENTER-TBI has shown is the difference in the patient outcomes among different hospitals and the continuous TBI process, with its consequences progressing over a long period, up to years.
Consecutive disability leads to the global economic and social burden of TBI [1], as seen in Figure 1.

The CENTER-TBI research team covered a wide range of information regarding TBI in their publications. They underlined the importance of using observational studies on greater levels, independently of the encountered difficulties on an organizational level. Every contributor to the international project of CENTER-TBI was involved in literature publications, including young researchers, thus leading to their scientific advancement. Moreover, several challenges and difficulties arose due to the variations among countries with regards to academic accreditation, letters of consent, and time for study approval. In some countries the duration for approval was of up to one and a half years [2].
Publications on CENTER-TBI
Over 200 publications regarding TBI were published based on CENTER-TBI. These covered gathered data from every stratum.
The prevention of cerebral venous thrombosis in severely injured TBI patients from the ICU stratum in 54 centers was analyzed, showing that pharmacological prophylaxis was used in more severely injured patients with a median hospital stay of 20 days, leading to a low incidence of venous thromboembolism. Furthermore, the most used pharmaceutical agents were low molecular weight heparins, although an important variability between countries was identified. The 6-month GOSE score done on TBI patients from the ICU showed improved scores on those who received venous thromboembolism prophylaxis during the hospital stay, proving its safety and possibility to improve the patient’s outcome at 6 months. Considering some study limitations, further studies regarding this matter should be made [3].
Another study emphasized the connection between serum biomarker levels, the type of injury, and its volume as revealed on imagery, namely cranial CT scan, in patients with all types of TBI regarding severity. GFAP, NSE, S100B, t-tau, NFL, and UCH-L1 were the researched biomarkers in the first 24 hours after injury.
The lesions assessed on imagery are highlighted in Figure 2.
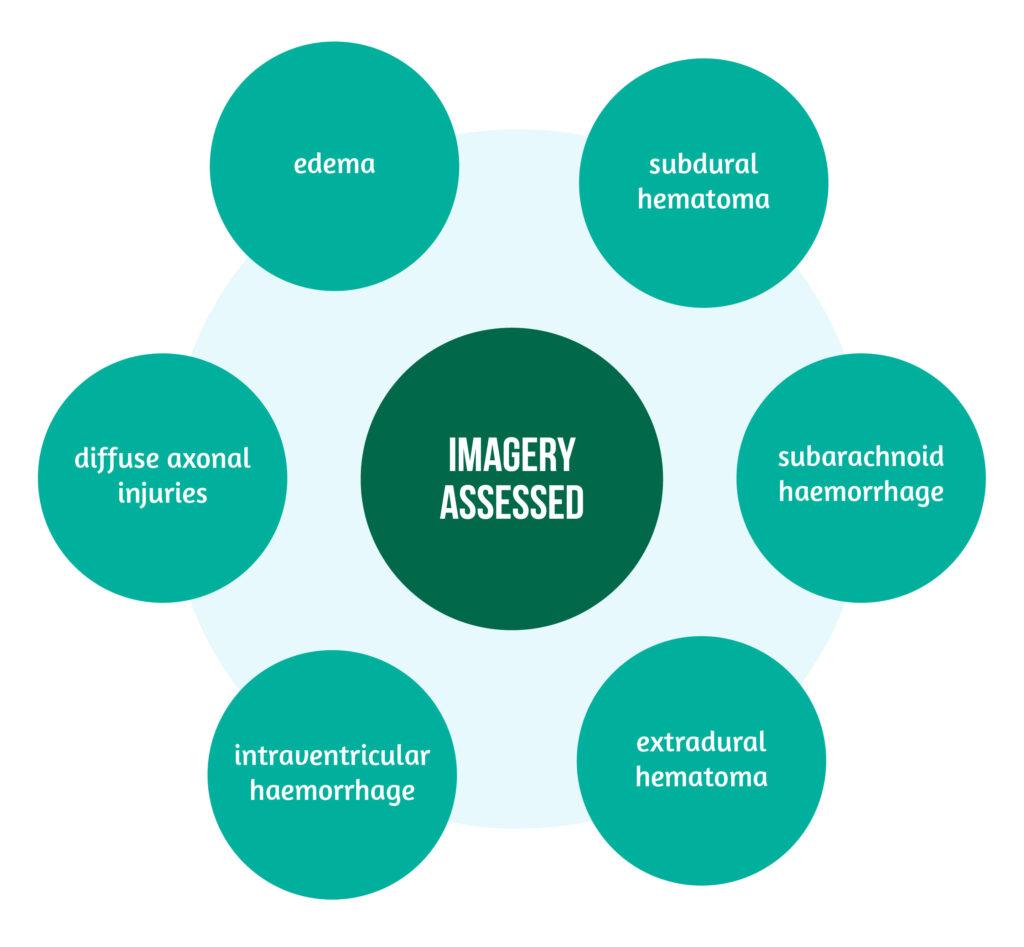
The elevation of biomarkers in the first 24 hours was correlated to the injury severity and the intracranial pressure [4].
Other studies based on CENTER-TBI
Other studies based on CENTER-TBI showed that patients suffering TBI secondary to low-energy falls represent an essential group of TBI patients, mainly of older age.
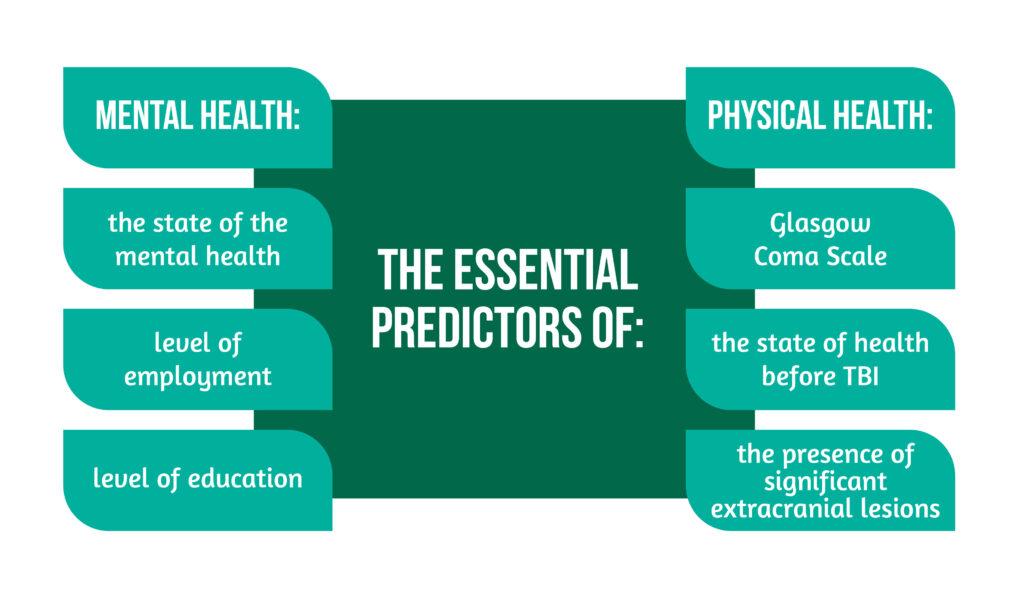
The mechanism of TBI involving high energy transfer does not entirely predict the mortality of patients with acute TBI, as well as the presence of intracranial injury [5]. By analyzing the outcome of TBI patients at 6 months with the help of SF-36v2 physical and mental health component scores and QOLIBRI (Quality of Life after Traumatic Brain Injury), the possibility of creating a prediction model for the Health-related Quality of Life after TBI was studied. From the sample of patients studied, almost two-thirds had mild TBI.
The essential predictors for mental health are highlighted in Figure 3 below [6]:
One last example of results published based on the data from CENTER-TBI is the implementation of the withdrawal of life-sustaining measures and its timing in severely affected TBI patients in ICU. The decision and the timing of the procedure represent a significant difficulty in medical practice, on the one hand, because if the decision is taken too early, the patient might lose the chance at a better outcome; on the other hand, if the decision is not taken in time, the consequences will be mirrored on the patient’s family, medical staff, as well as the healthcare system. Nevertheless, the study results revealed that the implementation of the withdrawal of life-sustaining measures was done early in around half of the studied population, <72 hours after injury, with a diagnosis of severe TBI involving the brainstem reflexes, without any impact regarding the geographical or center type.
CENTER-TBI on a global level
CENTER-TBI is part of the InTBIR project, a cooperation between the US National Institute of Neurological Disorders and Stroke (US NINDS), Canadian Institute of Health Research, and the European Commission, collecting data on TBI using mutually agreed data found in a Common Data Elements sheet developed by NINDS. Assuring followingly a match with FITBIR, as well as collaboration with different international initiatives regarding research on TBI, like TRACK-TBI (Transforming Research And Clinical Knowledge in Traumatic Brain Injury), CREACTIVE (Collaborative Research on ACute Traumatic brain Injury in IntensiVe care Medicine in Europe), ADAPT (Approaches and Decisions for Acute Pediatric TBI), PLAY GAME (Post-concussion Syndrome in youth: assessing the GABAergic effects of melatonin) or NEURO CARE (Neurocare: a clinical decision-making tool in youth mild TBI) [1].
This ambitious project mirrors the work, effort, and innovation of several centers in Europe and Israel combined, representing an example of collaboration, professionalism, and determination, contributing to the development of knowledge of TBI on all levels, from clinical and paraclinical aspects to the care and assessment of care.
References
- Maas AIR, Menon DK, Steyerberg EW, Citterio G et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI). Neurosurgery 2015, 76(1), 67–80. doi:10.1227/NEU.0000000000000575
- Maas AIR., Ercole A, De Keyser, V. et al. Opportunities and Challenges in High-Quality Contemporary Data Collection in Traumatic Brain Injury: The CENTER-TBI Experience. Neurocrit Care 2022. https://doi.org/10.1007/s12028-022-01471-w
- Huijben JA, Pisica D, Ceyisakar I, Stocchetti N et al. Pharmaceutical Venous Thrombosis Prophylaxis in Critically Ill Traumatic Brain Injury Patients. Neurotrauma Reports 2022.http://doi.org/10.1089/neur.2021.0037
- Whitehouse DP, Monteiro M, Czeiter E, et al. Relationship of admission blood proteomic biomarkers levels to lesion type and lesion burden in traumatic brain injury: A CENTER-TBI study. EBioMedicine. 2022;75:103777. doi:10.1016/j.ebiom.2021.103777
- Lecky FE, Otesile O, Marincowitz C, Majdan M et al. The burden of traumatic brain injury from low-energy falls among patients from 18 countries in the CENTER-TBI Registry: A comparative cohort study. PLOS Medicine 2021; 18(9): e1003761. https://doi.org/10.1371/journal.pmed.1003761
- Helmrich IRAR, van Klaveren D, Dijkland SA et al. Development of prognostic models for Health-Related Quality of Life following traumatic brain injury. Qual Life Res 2022; 31, 451–471 https://doi.org/10.1007/s11136-021-02932-z