Keywords: CENTER-TBI, TBI registry, TBI
Authors: Irina Vlad, Silvina Ilut
What is CENTER-TBI Project?
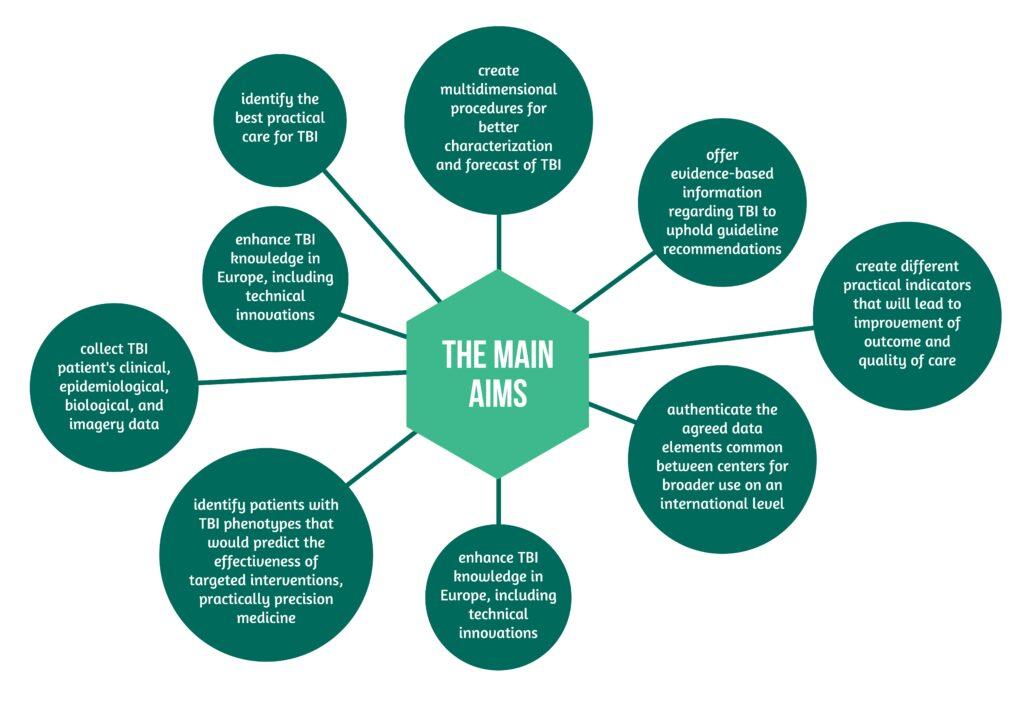
CENTER-TBI: the making-of the main TBI registry in Europe – The Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) core study and the CENTER-TBI registry comprise an essential part of the CENTER-TBI project, one of the most complex multinational and multidisciplinary projects endorsed by the European Union. The main aims are presented in Figure 1 below [1]:
TBI Explained
As important was the aim to introduce the patients and their families to the care of TBI in Europe, with emphasis on assuring information about the process of TBI using electronic platforms, social media, internet websites, as well as patients’ groups [1].
The variety in causes, mechanisms, pathological features, severity, treatment, and consequences makes TBI one of the most heterogeneous diseases, with complex intricacies and individualized effects on people. Furthermore, the heterogeneity of TBI can be seen in the type and severity of the lesions [1].
The type of lesions on imagery:
- undetectable lesions
- bleedings
- contusions
The severity of lesions:
- minor injuries (minor or transient symptoms with no consequences)
- lethal injuries

Project development and challenges
By using an integrated and homogenous approach on an international level, the project overcame part of the issues related to TBI’s heterogeneity by collaborating with researchers from different backgrounds and expertise. This considers and integrates today’s medical advances from genomics and neuroimaging to the analysis of different biomarkers that could prove helpful to the clinician as a method to characterize TBI. Furthermore, data gathering in different centers facilitated the comparison of practices regarding TBI, thus offering an opportunity for comparative effectiveness research and identification of the best practices with consecutive better outcomes. All of these lead to improved knowledge about TBI, ranging from prevention, diagnosis, and treatment, to monitoring, delivery, and assessment of the outcome.
The collected evidence will further represent a base for informative decisions of all the stakeholders involved in the management of TBI, including patients, clinicians, health systems representatives, and policymakers [1].
The established group of specialists managed to cover TBI’s heterogeneity from various points of view, including:
- clinical evolution
- paraclinical aspects (genes, different biomarkers, imagery)
- patient’s pathway [1].
Some impediments in the gathering of data and its analysis included: incorrectly inserted data, reluctance from professionals to insert the data, obtaining patient’s informed consent, to differences between centers, ranging, for instance, from technical aspects regarding imagery, logistics regarding biosamples or difficulties regarding data management [2].
The story and scope of CENTER-TBI registry in Europe
The CENTER-TBI Project was overseen by the two coordinators, Prof. Andrew I.R. Maas from the University Hospital Antwerp and Prof. David Menon from the University of Cambridge. They were joined by a management committee and national coordinators for each involved country. In addition, other organizational aspects, like logistics and assurance of qualitative data collection and the used platform, were covered by different organizations and institutions that collaborated in this project, including the Karolinska Institute International Neuroinformatics Coordinating Facility [1].
The study had several phases. The approximately one-year setup phase was followed by the recruitment phase, which should have lasted approximately eighteen months, in accordance to the rate and speed of the recruitment, and finally, by the follow-up phase at six months. Naturally, the cleaning, validation, and data analysis represented the last phase or step. The time frame for the project was planned to be between 2013 and 2017 [1].
The study was planned to take part in 80 centers from 22 countries in Europe (e.g., United Kingdom, France, Germany, Italy, Spain, Romania, Austria, and others) and Israel, for about 2 years. The core study should have covered around 5400 patients and the registry between 15000 and 25000 patients. Instead, between 2014 and 2017, data from 4509 patients were collected, from 18 countries (17 European countries, and Israel) covering the three elements of the TBI patient’s pathway, namely the emergency department, hospital admission, and intensive care unit [1,3].
The structure of CENTER-TBI registry in Europe
The study was composed of CENTER-TBI core data and the CENTER-TBI registry [1].
With clearly established inclusion and exclusion criteria, the registry covered TBI data from the participating centers, making between-centres comparisons as well as comparisons with national registries (when available). Furthermore, the core study gathered different biological samples – forming a biological depository – which were analyzed and used for additional research [1].
Data analysis was done to ensure better knowledge of TBI, from descriptive analytics to investigative research, aiming to identify new elements regarding TBI and understand its complexity. Machine learning techniques were also used [1].

The core study collected patient data for up to two years post-trauma, stratifying them into:
- patients seen in the emergency department and discharged at home
- patients hospitalized in different wards, except ICU (intensive care unit)
- patients sent directly to the ICU from the emergency department
Each stratum comprised a determined number of patients to ensure parity [1,3].
For diagnostic purposes, after signed informed consent, the biological materials were collected and analyzed [1].
Data collection from CENTER-TBI
The collected data covered at the different levels of patient care were:
- demographics
- socio-economic standing
- medical history
- injury history
- prehospital interventions
- imaging
- the pathway in the emergency room
- surgical interventions
- evolution of neurological status
- the motivation of several clinical decisions
- analysis of physicians’ care
- times of hospital admission
- discharge from the various wards
- patient’s prognosis
- discharge destination [1].
Furthermore, the patient’s blood was analyzed prospectively, with around two years of follow-up, according to the studied element, covering TBI protein biomarkers, genetic studies, the study of neurological imagery, and a detailed outcome assessment that was done by using different questionnaires.
A noteworthy mention is the fact that in some centers, where it was feasible and desired, more detailed data could be collected, from magnetic resonance imaging analysis to other biomarkers, as well as high-level ICU monitoring, including EEG or electrocorticographic, cerebral microdialysis, or brain oxygen monitoring [1].
References
1. Maas A IR., Menon DK, Steyerberg EW, Citterio G, Lecky F., Manley GT, Hill S, Legrand V, Sorgner A. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI). Neurosurgery 2015, 76(1), 67–80. doi: 10.1227/NEU.0000000000000575.
2. Burton A. The CENTER-TBI core study: The making-of. Lancet Neurol. 2017;16(12):958-959. doi:10.1016/S1474-4422(17)30358-73. CENTER-TBI: Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI), NIH US National Library of Medicine, ClinicalTrials.gov, Study Record Detail, published August 6,2021, https://clinicaltrials.gov/ct2/show/study/NCT02210221
3. CENTER-TBI: Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI), NIH US National Library of Medicine, ClinicalTrials.gov, Study Record Detail, published August 6,2021, https://clinicaltrials.gov/ct2/show/study/NCT02210221




